Despite the plethora of patient-facing digital tools in psychiatry (often called digital therapeutics), considerable challenges remain, in particular with respect to engagement. A 2019 study of 93 mental health apps with over 10 000 or more installs reported that less than 5% of users had opened the app after 2 weeks.
Reference Baumel, Muench, Edan and Kane1
A 2021 study offering a custom-designed mental health app offered to ∼50 000 college students saw only 117 individuals download it.
Reference Lattie, Cohen, Hersch, Williams, Kruzan and MacIver2
In contrast, engagement solutions are well established and multiple reviews have identified actionable steps ranging from co-design to gamification, workforce training to peer support.
Reference Cho, Olaye, Shandhi, Daza, Foschini and Dunn3
Yet in 2025, engagement is still a key issue and, by many accounts, has emerged as the single greatest challenge in digital mental health, hindering the intended outcome of any mental health intervention – to support positive outcomes for end users.
Reference Smith, Ward, Lambe, Ostinelli, Blease and Gant4
Why has so little headway been made on engagement despite the concerted effort and knowledge? In this perspective, we argue that the solution may lie closer than we expect – reframing engagement as a process rather than an outcome.
While solving the engagement problem is a multifaceted challenge, one core problem that remains is the lack of consensus on definitions, a well-known finding from numerous review papers.
Reference Linardon, Xie, Swords, Torous, Sun and Goldberg5,Reference Torous, Linardon, Goldberg, Sun, Bell and Nicholas6
Metrics such as minutes of app use, number of modules completed, percentage of activities completed and a myriad of other ways to measure or, better, quantify engagement proliferate across the literature. More subjective measures, such as perceived utility, perceived ease of use and perceived engagement itself, also abound. In this morass, objective measures of engagement have been justifiably criticised for failing to capture the meaning and value ascribed to engagement at any moment. Subjective metrics have also been questioned because of their limited generalisability and poor reliability.
But what if all these engagement metrics actually do precisely what they were designed to and deliver the correct information? Looking upstream at what they are measuring, in 2025, there are hundreds of thousands of health-related apps, virtual reality, computers and now even artificial intelligence products. We also know that, as in any developing field, some of these digital health tools work very well but many do not. Thus, successful engagement by a user may sometimes translate into positive benefits because they received an effective dose of an effective digital tool, but at other times may not because they received an adequate dose or an ineffective digital tool.
If many digital health tools lack effectiveness, tying engagement to each product’s efficacy blurs the line between user behaviour and product performance. This conflation makes it harder to interpret outcomes and obscures meaningful efficacy data. Did a digital health study fail because user engagement was too low or because the intervention was, in fact, not helpful to its users? Taken to one extreme, single-session interventions have risen in popularity because they can ignore the need for longer-term engagement. Taken to the other extreme, creating the ideal engagement environment for a new digital health tool can absorb so many resources and so much time that the efficiency and scalability of digital health are minimised.
Rather than considering adherence as a stand-alone problem, aligning engagement with outcomes offers a more productive solution. As in psychopharmacology, understanding how a drug engages a molecular receptor is critical to understanding its efficacy. The same applies to digital health: knowing how a tool engages specific mechanisms is also necessary. Taking the analogy further, different drugs can bind at the same receptor and have different effects because of other effects of the same drug on nearby receptors. In digital health, the same type of engagement will have a different impact depending on that tool’s other properties and mechanisms. Drug receptor binding and the resulting signal transduction is a complex field, but diving into its complexities has resulted in many novel treatments for cancers and infections. To date, digital health has resisted embracing that complexity.
Digital health and digital therapeutics have resisted this approach, as shown by the absence of standardised measures for engagement and the scarcity of replication or cultural adaptation studies.
Reference Naderbagi, Loblay, Zahed, Ekambareshwar, Poulsen and Song7,Reference Smith, Blease, Faurholt-Jepsen, Firth, Van Daele and Moreno8
An often-cited statistic is that it takes 10–15 years for a new drug to be developed, and 17 years for it to move from bench to bedside. Critics suggest that the advantage of digital health is that it can move much faster. However, digital health tools such as apps have been active and under design for at least 15 years. The few that have been cleared by the Food & Drug Administration have been critiqued, and not many would argue that advances in the space have outpaced classical drug development. So, moving from a product-focused and straightforward view of engagement towards a more nuanced and process view will be unlikely to hinder progress.
Progress in creating more effective apps can, and should be, accelerated by investing in the science of engagement. The field of digital health could benefit from genuine scientific progress and shared insights, by reframing engagement away from product-specific metrics to mechanistic-based pathways that can be tested, replicated and scaled. In developing new theories of engagement that can be empirically assessed, all parties benefit. Decoupling engagement from products means that companies can finally focus on building technologies that work without wondering whether users have received an adequate ‘dose’. Regulators can also better establish thresholds for efficacy by focusing on the unique mechanism of action for each technology. End users will benefit by understanding what each digital health technology can offer them for various levels of time, effort and engagement.
Despite incentives for more integrated research, a major challenge remains – a lack of ‘big picture’ thinking – and also the fragmented research and development landscape in which most teams still operate.
Reference Lilienfeld9
Subverting this approach, and rather than proposing a specific model or endorsing any metric, in November 2024 our authorship team met over 2 days to find consensus on the importance of the engagement process, regardless of the specific measurements of each step in that process.
Our team quickly realised that, with no standard definition for engagement in use, proposing one would not be productive. Rather than demanding a single measure, we propose a simple, four-step approach to explaining and sharing what the engagement process means in the context of each study, product or use case. Each step in the model is well known, but the combination of steps forces redefining of engagement from an outcome to a process. With better sharing of engagement process metrics and transparency on their successes/failures, the field will be able to identify those use cases and theories around which to unify.
The resulting model is simple in its four steps, as shown below and in Fig. 1.
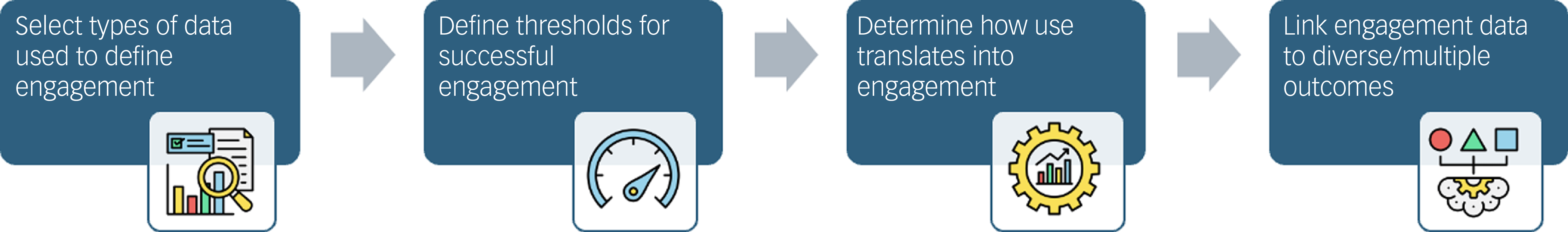
Fig. 1 A schematic of the four-step model.
Step 1: The types of data used to define engagement measurement are predefined and will be recorded throughout the study. The choice is up to the team, and may include classical measures such as screen time, subjective scales, novel outcomes or some combination thereof.
Step 2: A threshold for successful engagement is predefined. The choice is also up to the team, and may be a percentage cut-off for modules completed, a particular score of surveys, etc., but must be pre-justified for why it matters. The goal is that the field will unify around productive and informative thresholds.
Step 3: The team proposes how, for those users reaching that threshold, use at that rate may translate into engagement. For example, completing different module percentages could suggest different engagement levels. This step has an overlap with step 2, the goal being to associate the measure of separate use from engagement.
Step 4: The team links the engagement data to diverse/multiple outcomes; these need not be only efficacy goals or changes in symptoms.
This model aims to trace the transformation of usage data metrics into engagement metrics, and those engagement metrics into meaningful outcomes for digital health technology. By making explicit how engagement is defined and what it is doing, we can focus on its downstream impacts, namely improving outcomes for their intended users. This approach also focuses on mechanisms of action around how engagement may be acting, translating the fractured language of engagement today into more scientifically testable definitions that can guide future research. In allowing for different types of data, thresholds, considerations of engagement, specific biomarkers and resulting outcomes, the goal is to enable successful approaches to be shared, replicated and scaled. This approach enables any team or company to maintain their current definitions and metrics of engagement, but extends those metrics by placing them in the context of a process from raw data to clinical impact. This approach does not replace the need for hybrid implementation studies, but rather complements these by making engagement processes more tangible and transparent.
